
 |
Explain the instability of metals
Discuss the factors that can trigger corrosion
Explain the chemistry of corrosion
Review the definition of acidity
Compare corrosion reactions of some common metals
This Module consists of three Web pages of required reading. The pagination is visible at the bottom of each page with direct links to adjacent pages.
Additional information can be found in sections 2.1, 2.2, 2.3, and 2.4 of the reference textbook (Corrosion Engineering: Principles and Practice).
The driving force that causes metals to corrode is a natural consequence of their temporary existence in metallic form. To reach this metallic state from their occurrence in nature in the form of various chemical compounds (ores), it is necessary for them to absorb and store up for later return by corrosion, the energy required to release the metals from their original compounds.
Compare the energy required to produce one metric ton of magnesium from its oxide to the energy required to convert enough copper oxide to produce one ton of metallic copper.
Discuss the energy values presented in the Table shown on the page describing why metals corrode in relation to the order in which metals and associated alloys appeared in the history of mankind.
When discussing the ionic content of an aqueous medium, the question often arises as to how acid (or alkaline) is the solution. Quite simply, this refers to whether there is an excess of H+ (hydrogen) or
The pH may be measured with a meter or calculated if certain parameters are established. Water itself dissociates to a small extent to produce equal quantities of H+ and
![]()
pH , originally defined by Danish biochemist Søren Peter Lauritz Sørensen in 1909, is a measure of the concentration of hydrogen ions. The term pH was derived from the manner in which the hydrogen ion concentration is calculated, it is the negative logarithm of the hydrogen ion (H+) concentration:
![]()
where log is a base-10 logarithm and aH+ is the activity (related to concentration) of hydrogen ions. The "p" in Equation stands for the German word for "power", potenz, so pH is an abbreviation for "power of hydrogen".
A solution is made up to contain 0.01 M HCl. What is its pH?
A solution is made up to contain 0.01 M NaOH. What is its pH?
A higher pH means there are fewer free hydrogen ions, and that a change of one pH unit reflects a tenfold change in the concentrations of the hydrogen ion. For example, there are 10 times as many hydrogen ions available at pH 7 than at pH 8. The pH scale commonly quoted ranges from 0 to 14 with a pH of 7 considered to be neutral.
Substances with a pH less that 7 are considered to be acidic and substances with pH equal to or greater than 7 to be basic or alkaline. Thus, a pH of 2 is very acidic and a pH of 12 very alkaline. However, it is technically possible to have very acidic solutions with a pH lower than zero and concentrated caustic solutions with a pH greater than 14. Such solutions are in fact typical of many ore extracting processes that require the digestive power of caustics and acids.
Low pH acid waters accelerate corrosion by
supplying hydrogen ions to the corrosion process. Although even absolutely pure water contains some free hydrogen ions, dissolved carbon dioxide (CO2) in the water can increase the hydrogen ion concentration. Dissolved CO2 may react with water to form carbonic acid (H2CO3) as shown in equation.
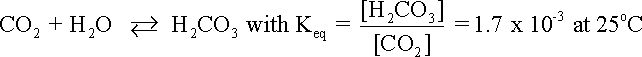
where Keq is the reaction equilibrium expressed as a ratio.
Carbonic acid subsequently dissociates in bicarbonate and carbonate ions as expressed respectively in the following equations:
![]()
![]()
A solution contains a mixture of sodium bicarbonate (0.05 M) and sodium carbonate (0.2 M).What is its pH?
Care must be taken when quoting and using the dissociation constant in equation. This equilibrium value is correct for the H2CO3 molecule, and shows that it is a stronger acid than acetic acid or formic acid as might be expected from the influence of the electronegative oxygen substituent. However, carbonic acid only exists in solution in equilibrium with carbon dioxide, and so the concentration of H2CO3 is much lower than the concentration of CO2, reducing the measured acidity. The equation may be rewritten as follows:
![]()
Even more acidity is sometimes encountered in mine waters and in water contaminated by industrial wastes. Many salts added to an aqueous system also have a direct effect on the pH of that mixture through the following process of hydrolysis shown here for the addition of ferric ions to water:
![]()
In this particular example the equilibrium is established between ferric ions, water, ferric hydroxide or Fe(OH)3 and the acidity of the water. This particular example is quite useful to explain the severity of a situation that can develop in confined areas such as corrosion pitting and crevices.
| (previous) | Page 1 of 3 | (next) |
See also CCE 513: Corrosion Engineering