
 |
When one of the reactions is limited by the rate of transport of the reactant to the metallic surface being corroded, the situation increases in complexity as illustrated in the polarization plot of the system in the following Figure. The system represented here is similar to the previous one, i.e. pH of five at 25oC, with the exception that the environment is aerated and stagnant. In this situation the reduction of oxygen shown in equation now becomes possible as a second cathodic reaction. (reference)
![]()
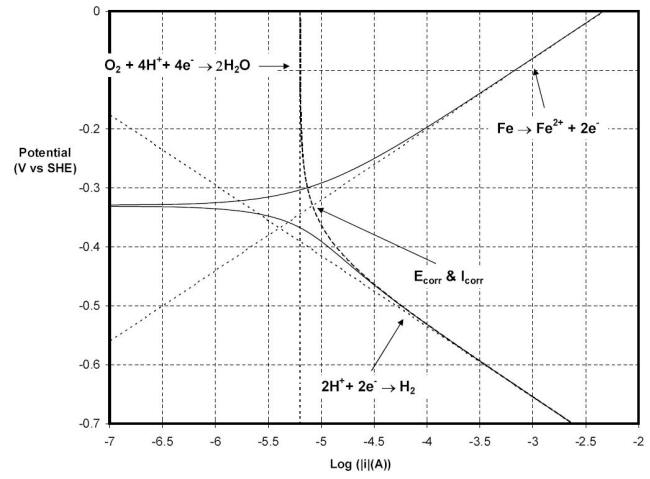
Polarization behavior of carbon steel in a stagnant aerated solution maintained at 25oC and a pH of five
In order to model the polarization plot, the total cathodic current corresponding to the sum of the currents of the hydrogen reaction and oxygen reduction has to be balanced by the single anodic current. The intercept where the opposing currents are balanced occurs at an Ecorr of -0.33 V vs. SHE and, since the surface area is still one cm2, an icorr of 8.2 mA cm-2 or 0.1 mm y-1. It should be noted that the current for the reduction of oxygen is constant across the potential range shown in the previous Figure and has the value of 6.3 mA cm-2.
The reduction of oxygen depends, among other factors, on the level of agitation of the environment. If the limiting current of this reaction is now increased by a tenfold factor to reach the value of 63 mA cm-2 a new situation emerges as depicted in the Figure 5.18. The marked positive shift of Ecorr, now -0.224 V vs. SHE, is accompanied by a marked increase in current density, now of 63 mA cm-2 or 0.8 mm y-1.
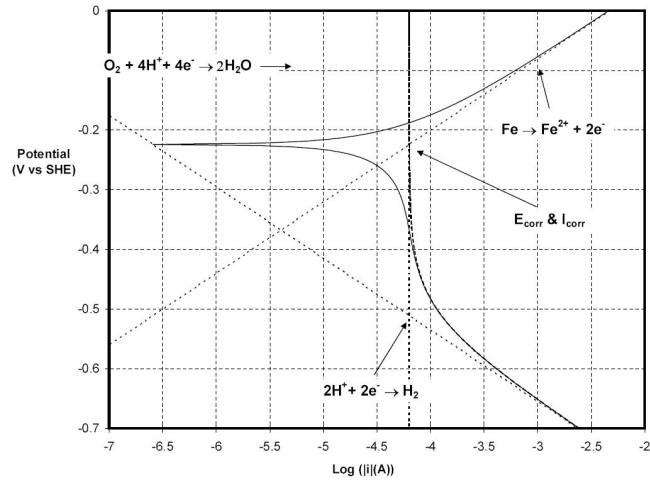
Polarization behavior of carbon steel in an agitated aerated solution maintained at 25oC and a pH of five
| (previous) | Page 10 of 10 | (next Module) |