
 |
When reporting electrochemical potential measurements, it is always important to indicate which reference half-cell was used to carry out the work. This information is required to compare these measurements to similar data that could have been obtained using any other reference half-cells. The scheme presented in the following Figure provides a graphical representation to help visualize some of the information listed here. (reference)
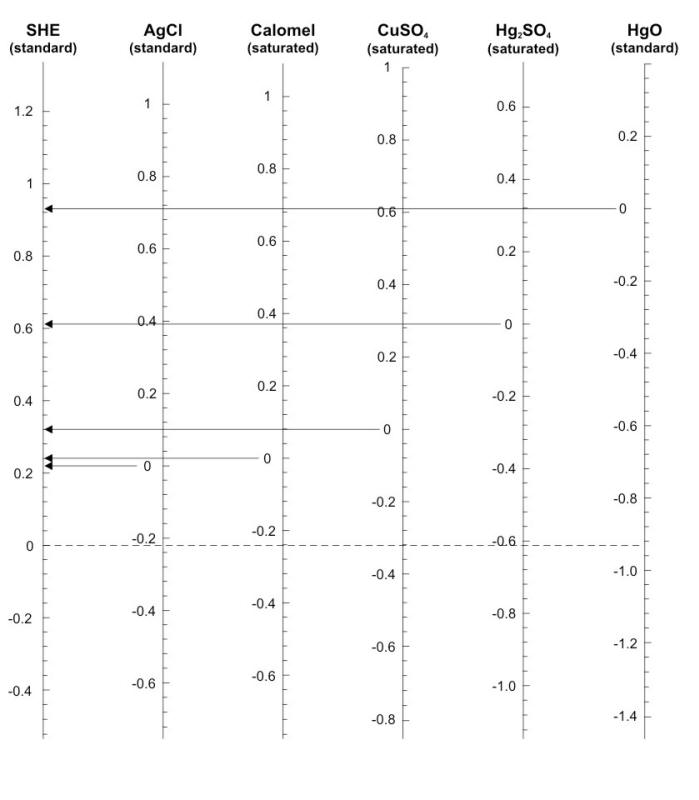
Graphical scheme to compare potentials of the most commonly used reference electrodes
We can take the case of a measurement of the potential of a steel pipe buried in the ground, using a saturated copper-copper sulfate reference electrode (CCSRE). This might show a potential of -0.700 V measured in this way. To convert this potential to a value on the scale in which the hydrogen electrode has a potential of zero, it is necessary to add 0.318 volt to the potential that was measured, making it - 0.382 volt vs. SHE.
What does a measured potential value of 0.8 V vs. SHE would be if the potential had been measured with a saturated silver chloride electrode? ... with a saturated copper sulfate electrode?
| (previous) | Page 6 of 17 | (next) |