Intergranular Corrosion |

The microstructure of metals and alloys is made up of grains, separated by grain boundaries. Intergranular corrosion is localized attack along the grain boundaries, or immediately adjacent to grain boundaries, while the bulk of the grains remain largely unaffected. This form of corrosion is usually associated with chemical segregation effects (impurities have a tendency to be enriched at grain boundaries) or specific phases precipitated on the grain boundaries. Such precipitation can produce zones of reduced corrosion resistance in the immediate vicinity.
The attack is usually related to the segregation of specific elements or the formation of a compound in the boundary. Corrosion then occurs by preferential attack on the grain-boundary phase, or in a zone adjacent to it that has lost an element necessary for adequate corrosion resistance - thus making the grain boundary zone anodic relative to the remainder of the surface. The attack usually progresses along a narrow path along the grain boundary and, in a severe case of grain-boundary corrosion, entire grains may be dislodged due to complete deterioration of their boundaries. In any case the mechanical properties of the structure will be seriously affected.
A classic example is the sensitization of stainless steels or weld decay. Chromium-rich grain boundary precipitates lead to a local depletion of Cr immediately adjacent to these precipitates, leaving these areas vulnerable to corrosive attack in certain electrolytes. Reheating a welded component during multi-pass welding is a common cause of this problem. In austenitic stainless steels, titanium or niobium can react with carbon to form carbides in the heat affected zone (HAZ) causing a specific type of intergranular corrosion known as knife-line attack. These carbides build up next to the weld bead where they cannot diffuse due to rapid cooling of the weld metal. The problem of knife-line attack can be corrected by reheating the welded metal to allow diffusion to occur.
Many aluminum base alloys are susceptible to intergranular corrosion on account of either phases anodic to aluminum being present along grain boundaries or due to depleted zones of copper adjacent to grain boundaries in copper-containing alloys.Alloys that have been extruded or otherwise worked heavily, with a microstructure of elongated, flattened grains, are particularly prone to this damage.
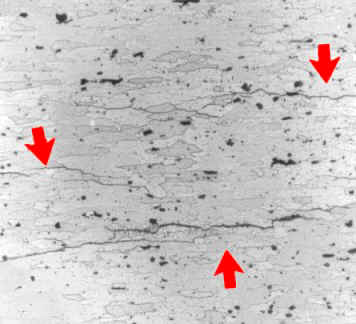
Intergranular corrosion of a failed aircraft component made of 7075-T6 aluminum (picture width = 500 mm)
| (previous) | Page 13 of 23 | (next) |