Pitting Corrosion |
Pitting corrosion is a localized form of corrosion by which cavities or "holes" are produced in the material. Pitting is considered to be more dangerous than uniform corrosion damage because it is more difficult to detect, predict and design against. Corrosion products often cover the pits. A small, narrow pit with minimal overall metal loss can lead to the failure of an entire engineering system. Pitting corrosion, which, for example, is almost a common denominator of all types of localized corrosion attack, may assume different shapes.
Suppose . . .that a small cavity exists in the surface of the metal into which oxygen cannot diffuse quickly. A current will be produced between the unaerated area within the cavity, which will become anodic, and the aerated part of the surface outside, which will be the cathode; soluble salt will be formed at the anodic surface within the cavity, but this will not, of course, interfere with further anodic attack. At the mouth of the cavity where the soluble metallic salt from the interior mixes with the alkali from the cathodic part outside, hydroxide may be precipitated, but it will not put a stop to the anodic attack proceeding within. Since the rate of attack is determined by the supply of oxygen to the whole surface outside the pit, and since it is all concentrated on the small area within the pit, the rate at which the corrosion bores into the metal will be very great; . . . U.R. Evans, 1924
Pitting corrosion can produce pits with their mouth open (uncovered) or covered with a semi-permeable membrane of corrosion products. Pits can be either hemispherical or cup-shaped
Pitting is initiated by:
- Localized chemical or mechanical damage to the protective oxide film; water chemistry factors which can cause breakdown of a passive film are acidity, low dissolved oxygen concentrations (which tend to render a protective oxide film less stable) and high concentrations of chloride (as in seawater)
- Localized damage to, or poor application of, a protective coating
- The presence of non-uniformities in the metal structure of the component, e.g. nonmetallic inclusions.
Theoretically, a local cell that leads to the initiation of a pit can be caused by an abnormal anodic site surrounded by normal surface which acts as a cathode, or by the presence of an abnormal cathodic site surrounded by a normal surface in which a pit will have disappeared due to corrosion.
|
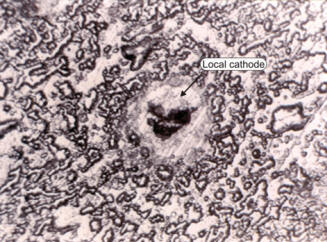
In the second case, post-examination should reveal the local cathode, since it will remain impervious to the corrosion attack as in the picture of an aluminum specimen shown on the right. Most cases of pitting are believed to be caused by local cathodic sites in an otherwise normal surface. |
|
Apart from the localized loss of thickness, corrosion pits can also be harmful by acting as stress risers. Fatigue and stress corrosion cracking (SCC) may initiate at the base of corrosion pits.
One pit in a large system can be enough to produce the catastrophic failure of that system. An extreme example of such catastrophic failure happened recently in Mexico, where a single pit in a gasoline line running over a sewer line was enough to create great havoc to a city, killing 215 people in Guadalajara.
Why would pitting corrosion be much more prone to provoke a catastrophic failure than uniform corrosion generally does?
Some definitions
-
Pitting: corrosion of a metal surface, confined to a point or small area, that takes the form of cavities.
-
Pitting factor: ratio of the depth of the deepest pit resulting from corrosion divided by the average penetration as calculated from weight loss.
-
Pitting resistance equivalent number (PREN): an empirical relationship to predict the pitting resistance of austenitic and duplex stainless steels. It is expressed as PREN = Cr + 3.3 (Mo + 0.5 W) + 16N.
| (previous) | Page 4 of 23 | (next) |
Some examples
-
Pitting corrosion causing leaks: pitted pot leaking water.
-
Sewer explosion due to corrosion: example of corrosion damages with shared responsibilities.
-
Corrosion pit shapes:pits with their mouth open or covered with corrosion products.
-
Pitting corrosion data:incidents of aircraft and helicopters.


Connect with us
Contact us today