
 |
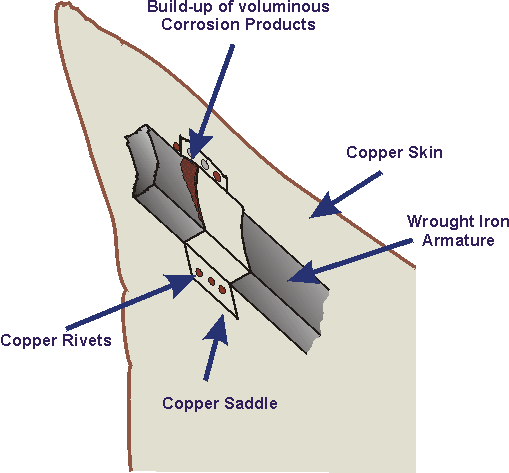
The galvanic reaction between iron and copper was originally mitigated by insulating copper from the iron framework using an asbestos cloth soaked in shellac. However, the integrity and sealing property of this improvised insulator broke down over the many years of exposure to high levels of humidity normal in a marine environment. The insulating barrier became a sponge that kept the salted water present as a conductive electrolyte, forming a crude electrochemical cell as and Volta had discovered a century earlier. The formation of expanded material that followed was typical of confined situations found in crevice corrosion.
Other Statue of Liberty topics: Auguste Bartholdi, Construction, Copper, Corrosion, History, Head, Introduction, Model, Picture, Postcard, Restoration, Symbolism
Other landmarks: Christ the Redeemer, Colossus, Delhi pillar, Eiffel tower, Golden Gate bridge, Great Buddha, Guggenheim Museum (Bilbao), Guggenheim Museum (NYC), Normandy bridge, Oresund crossing, Quebec Bridge, Statue of Liberty, Thames Barrier, Titanic, Tower of the Orologio