
 |
One of the common ways of generating hydrogen in a laboratory is to place zinc into a dilute acid, such as hydrochloric or sulfuric. When this is done, there is a rapid reaction in which the zinc is attacked or “dissolved” and hydrogen is evolved as a gas.

Rapid evolution of hydrogen bubbles during the corrosion of a zinc strip in a 1 M HCl acid solution
These reactions are described in the following equations to:
![]()
![]()
These equations are the chemical shorthand for the statement: One zinc atom + two hydrochloric acid molecules dissociated as ions H+ and Cl- and becomes one molecule of zinc chloride in the first equation and written as a soluble salt in the form of Zn2+ and Cl- ions in the second equation + one molecule of hydrogen gas which is given off as indicated by the vertical arrow. It should be noted that the chloride ions do not participate directly in this reaction, although they could play an important role in real corrosion situations.
Similarly, zinc combines with sulfuric acid to form zinc sulfate (a salt) and hydrogen gas as shown in the following equations:
![]()
![]()
Note that each atom of a substance that appears on the left-hand side of these equations must also appear on the right-hand side. There are also some rules that denote in what proportion different atoms combine with each other. As in the preceding reaction, the sulfate ions that are an integral part of sulfuric acid do not participate directly to the corrosion attack and therefore one could write these equations in a simpler form:
![]()
Many other metals are also corroded by acids often yielding soluble salts and hydrogen gas as shown in Equations and for respectively iron and aluminum:
![]()
![]()
Note that zinc and iron react with two H+ ions, whereas aluminum reacts with three. This is due to the fact that both zinc and iron, when corroding, each lose two electrons and display two positive charges in their ionic form. They are said to have a valence of +2 or II, whereas aluminum loses three electrons when leaving an anodic surface and hence displays three positive charges and is said to have a valence of +3 or III. Some metals have several common valences, others only one. The following Figure shows Some of the oxidation states found in compounds of the transition-metal elements.
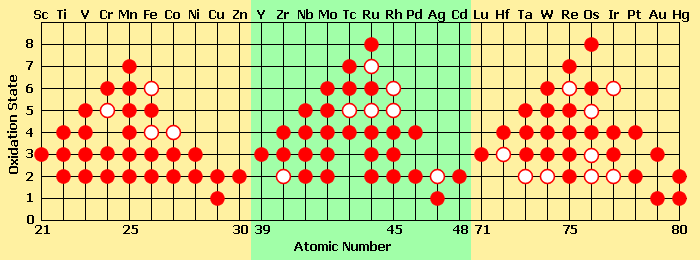
Oxidation states found in compounds of the metalic elements. A solid circle represents a common oxidation state, and a ring represents a less common (less energetically favorable) oxidation state
| (previous) | Page 2 of 3 | (next) |