
 |
The Nernst equation was named after the German chemist Walther Nernst who established very useful relations between the energy or potential of a cell to the concentrations of participating ions. This equation can be derived from the equation linking free energy changes to the reaction quotient (Qreaction): (reference)
![]()
where, for a generalized equation of the form:
![]()
The capital letters A, B, M and N in equation represent respectively the reactants and products of a given reaction while the small letters represent the coefficients required to balance the reaction.
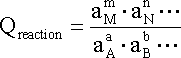
At equilibrium, DG = 0 and Qreaction
corresponds to the equilibrium constant (Keq) described earlier.
In the case of an electrochemical reaction, substitution of
the relationships DG = -nFE and D
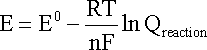
Combining constants at 25oC (298.15 K) gives the simpler form of the Nernst equation for an electrode reaction at this standard temperature:
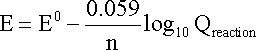
In this equation, the electrode
potential (E) would be the actual potential difference across a cell containing
this electrode as a half-cell and a standard hydrogen electrode as the other half-cell.

Some of the species that take part these electrode reactions are pure solid compounds and pure liquid compounds. In dilute aqueous solutions, water can be treated as a pure liquid. For pure solid compounds or pure liquid compounds, activities are constant and their values are considered to be unity. The activities of gases are usually taken as their partial pressures and the activities (ai) of solutes such as ions are the product of the molar concentration and the activity coefficient of each chemical species (i):
![]()
The activity coefficient (gi) in equation can be a complex function highly dependent on a multitude of variables often difficult to even estimate. For this reason it is usually convenient to ignore (gi) and use the concentration term [i] as an approximation of ai.
| (previous) | Page 4 of 17 | (next) |