
 |
Snow fighting has a long history. However, the first use of salt for deicing roads can only be traced back to the 1930’s and it was not until the 1960’s that the use of salt in conjunction with plowing became widespread after winter maintenance personnel learned of its effectiveness. The following Figure presents the usage made of deicing salt or rock salt (mostly sodium chloride) in the U.S. between 1940 and 2005 . Initially confined to the ‘snowbelt’, ice fighting has become a priority activity in the ‘sunbelt’ too.
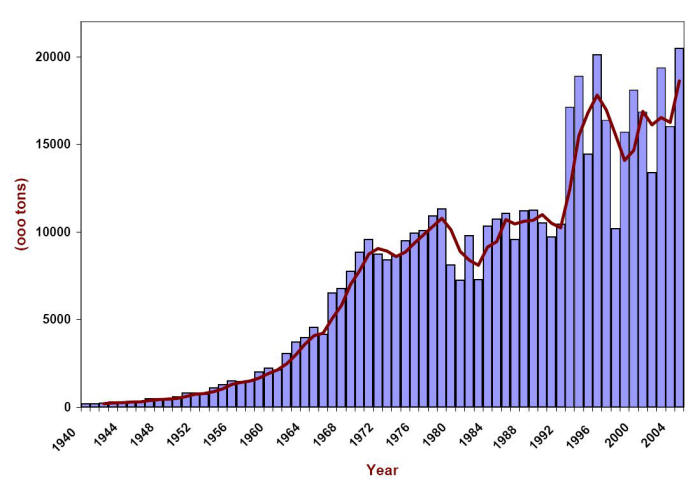
Usage of rock salt for deicing in the U.S. in thousands of tons. (Data from the Salt Institute http://www.saltinstitute.org/)
There is a vast international experience in effective snow fighting and the efficient use of deicing salts. A 1972 study by Paul J. Claffey presented to the Highway Research Board (now Transportation Research Board) concluded that the roughness of road ice and slippage of wheels can result in an increase in fuel consumption around 35% in averages and as much as 50% when there is 5 cm of snow on the road. A 1976 report by the Institute for Safety Analysis (TISA) listed the following cost benefits (reported here using 1976 prices and rates) of using salt in the U.S. to deice highways:
Reduces wages lost due to lateness to work by $7.6 billion
Saves $3 billion in wage loss because of absenteeism
Reduces production losses by $7 billion
Reduces losses in goods shipment by $600 million
Saves 1.4 to 4.5 billion liters of fuel
Has an 18:1 benefit/cost ratio
A study published in 1993 concluded that ‘As a winter maintenance service, deicing pays for itself within the first 25 minutes after the first hour that salt is spread on two-lane highways. Then, during the first four hours after the hour of application of salt, the direct road user benefits were $6.50 for every $1.00 spent on direct maintenance costs for the operation.’ The study found that costs related to accidents, including medical expenses, emergency services, workplace costs, travel delay, property damage, and administration and legal expenses decrease by 88% after the application of deicing salt. The following Figure illustrates the results of two studies of traffic accident rates carried out in two different countries (Germany and the U.S.) before and after salt spreading,
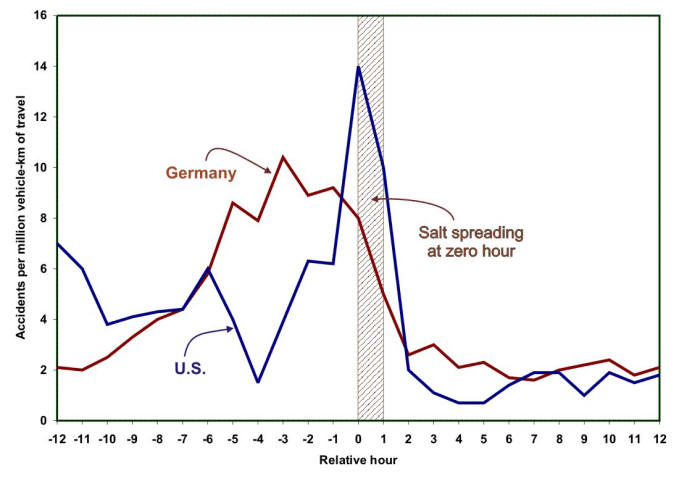
Statistics revealing the number of accidents before and after applying deicing salts.
Use of salt, in conjunction with a good plowing program, is the fastest and most efficient means of snow and ice removal. The use of abrasives requires at least seven times more material to treat a given distance of roadway. Therefore, it takes seven loads and seven round trips to the loading point, compared to just one for salt, resulting in a greater use of fuel, increased manpower and more time to treat roads during a storm. Studies by the Salt Institute have determined that a loaded salt truck, spreading at the generally accepted rate of 140 kg per two-lane km for general storm conditions, can treat a 36 km stretch of roadway, traveling a total of 72 km. A sand truck requires seven loads, must travel a total distance of 300 km to treat the same section of road and that truck requires four times more fuel. In more ways than one, salt used in snow and ice control contributes to energy savings.
The massive spreading of salt on roads and highways unfortunately has also some serious negative effects. Besides environmental concerns, one of the major criticisms of salt use for deicing is its contribution to corrosion of metal in steel bridges, road vehicles, reinforced concrete (bridge decks, parking garages), and any other metallic objects in close proximity to roads and highways (lampposts, statues, etc.).
Although another effective but less corrosive deicing agent is commercially available (calcium magnesium acetate or CMA), its price is apparently too high for wide usage. One ton of CMA costs $300-600 as opposed to $20-70 for rock salt. The use of CMA has thus been confined to areas where corrosion could cause critically important damage such as for airport winter maintenance. It can therefore be expected that the road environment will likely remain corrosive well into the future.
Maps are powerful tools for communicating information related to geographical landscapes and corrosivity maps of various countries have thus been drawn to illustrate the corrosion severity of the regions of these countries as it varies from coastal areas to deserts and from rural to heavily industrial locations. One of the very first such maps has been produced to summarize many years of results obtained by exposing bare steel coupons attached to different vehicles in the north-eastern U.S. and Canada. This corrosivity map is shown in the following Figure in which the snowbelt region is circled. The higher level of vehicle corrosion in the snowbelt region when compared to adjacent non-marine regions can only be attributed to the use of deicing salts.
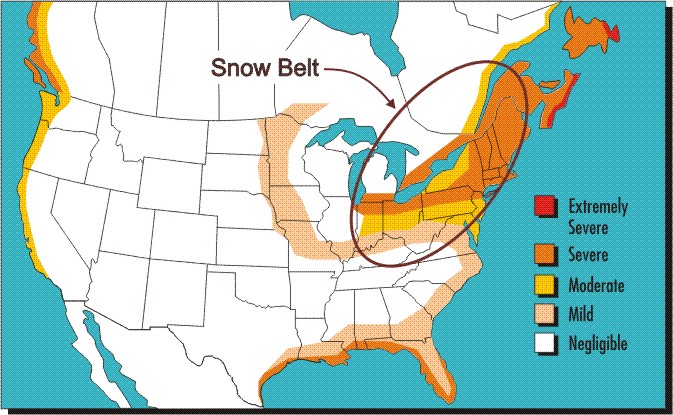
Corrosivity map of North America showing the particular aggressiveness of the Snowbelt region.
The impact of salts on protective coatings is widely recognized. Any breach or holiday in the coating will let salts reach the metallic substrate and initiate a very aggressive environment that in turn will force the coating to blister and peel off as shown in the following Figures.

Blistering and peeling of protective coating subjected to regular deicing salt application.
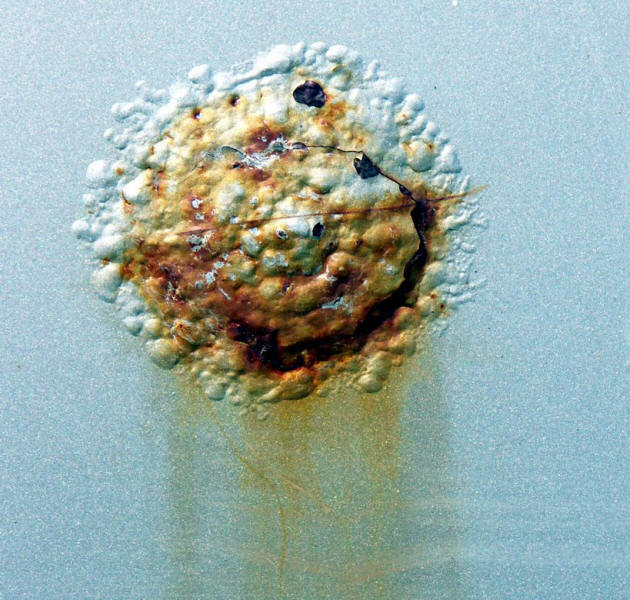
Filiform corrosion and paint blistering accelerated by deicing salt.
The effect of deicing salts extends much beyond the immediate vicinity where the salts are spread because these salts can travel as aerosol particles generated by the traffic circulation. The lifetime of any particular particle depends on its size and location. Studies of the migration of aerosols inland of a sea coast have shown that typically the majority of the aerosol particles are deposited close to the shoreline (typically 400 to 600 m) and consist of large particles (>10µm diameter), which have a short residence time and are controlled primarily by gravitational forces. Some studies have also indicated that there is a strong correlation between wind speed and the deposition and capture of aerosols.
The following Figure summarizes the results of a study using standard corrosion coupons deployed on a pedestrian walkway across a well traveled road during the winter months of a snowbelt city (Kingston, Ontario, Canada).

Results of a study investigating the transport and effects of deicing salt laden aerosols.
These results clearly indicate that the corrosion rates (% mass loss) while being highest closer to the ground (at the bottom of the pillars) are still appreciable many meters above the traffic level. For comparison, similar measurements made in non-trafficked areas of the same city typically showed corrosion rates fifty times smaller.
| (previous) | Page 5 of 8 |
(next) |