
 |
The potential difference across an electrochemical cell is the potential difference measured between two electronic conductors connected to the electrodes. In the external circuit, the electrons will flow from the most negative point to the most positive point and, by convention, the current will flow in the opposite direction. Since the electrode potential can be either positive or negative, the electrons in the external circuit can also be said to flow from the least positive electrode to the most positive electrode. A voltmeter may be used to measure the potential differences across electrochemical cells but cannot measure directly the actual potential of any single electrode. Nevertheless, it is convenient to assign part of the cell potential to one electrode and part to the other. (reference)
There are several potential bench marks in common use, but the most ancient is the half-cell in which hydrogen gas is bubbled over a platinum electrode immersed in a solution having a known concentration of hydrogen ions.
This historically important reference electrode is called the standard hydrogen electrode (SHE) if a standard solution of acid is used. "By definition" the equilibrium potential of this electrode is zero at any temperature." The SHE is also called by many "normal hydrogen electrode" (NHE) in reference to a solution containing one equivalent of protons. Strictly speaking, one must use unit activity rather than concentration of hydrogen ions and unit fugacity rather than unit pressure of hydrogen gas.
However, the SHE can be somewhat inconvenient to use because of the need to supply hydrogen gas. Therefore, other reference electrodes are much preferred for practical considerations. The potential difference across a reversible cell made up of any electrode and a SHE is called the reversible potential of that electrode, E.If this other electrode is also being operated under standard conditions of pressure and concentration, then the reversible potential difference across the cell is the standard electrode potential E0 of that electrode.
Tables of standard electrode potentials in either alphabetical order or by decreasing potential values can be obtained if any one electrode, operated under standard conditions, is designated as the standard electrode or standard reference electrode with which other electrodes can be compared.
Rank the following ions in order of their thermodynamic ease of plating out of a solution: Cu2+, Co2+, Fe2+, Fe3+, Na+, Pb2+, Cu+
Since an electrochemical reaction can be written either as an oxidation or a reduction causing confusion in relation to the sign of the potential of that reaction, a convention was adopted in
Rank the following elements in order of their thermodynamic ease of being oxidized in solution: Hg, Al, Fe, Au, Cr, Zn, Ag, Mg
Using standard potentials and molarity for ion concentrations calculate the open circuit potential of the following electrochemical reactions (balance the equations with water related chemical species when necessary, i.e. H+, OH- and H2O):
H2O2 + Ni → H2O + Ni2+
H2O + Mg2+ → H2O2 + Mg
Ni + PbO2 → Pb2+ + Ni2+
| (previous) | Page 3 of 17 | (next) |
Standard-state reduction half-cell potentials in alphabetical order
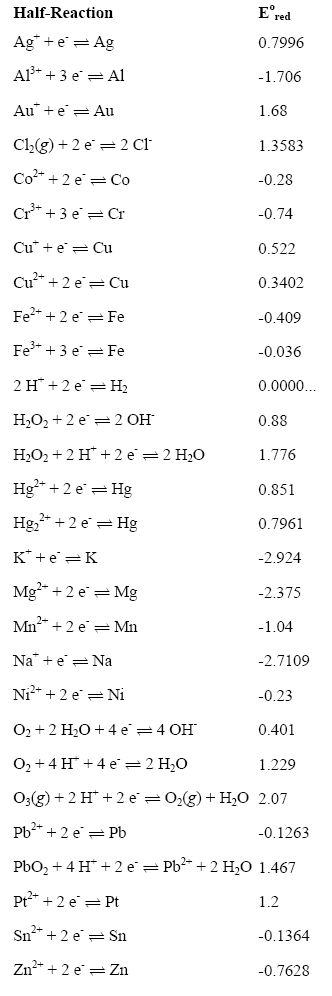
Standard-state reduction half-cell potentials by decreasing order of potential.
