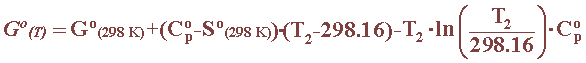|
The free energy of a substance, for which heat capacity data are available, can be calculated as a function of temperature using the following equation:
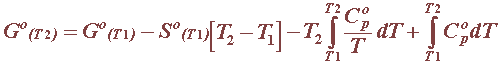
For pure substances, i.e. solids, liquids and gases, the heat capacity is often expressed as a function of the absolute temperature, as in this table for all pure substances involved in reference electrodes:
![]()
For ionic substances, one has to use another method, such as proposed by Criss and Cobble in 1964, to obtain the heat capacity, provided the temperature does not rise above 200oC. The expression of the ionic capacity makes use of absolute entropy values and the parameters a and b contained, as in this table for the ionic substances involved in reference electrodes:
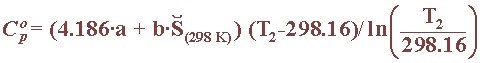
By combining these previous two equations, one can obtain the free energy, at any given temperature, of all the chemical species involved in a given reaction: