Nitric Acid
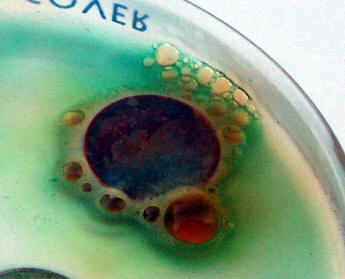
Nitric acid is considered to be a strong acid and oxidant. As can be seen in the picture shown above, nitric acid is particularly aggressive to most metals, and even more so with copper. While the green soluble copper nitrate spreads away fro the reacting site, the deep red-brown nitrogen dioxide gas that is freed according to the following equation:

Jabir Ibn Hayyan (c. 721, Tus, Khurasan, Iran – 815, Kufa, Iraq), full name: Abu Musa Jabir Ibn Hayyan Al-Azdi (أبو موسى جابر بن حيان الأزدي), was an Arabian born in Persia. Referred to in Western contexts by the Latinized form of his given name (Jabir), Geber, also known as the Father of Chemistry, because he was the first to scientifically systematize chemistry.
Jabir is also credited with the invention and development of several chemical instruments that are still used today, such as the alembic, which made distillation easy, safe, and efficient. By distilling various salts together with sulfuric acid, Jabir discovered hydrochloric acid (from salt) and nitric acid (from saltpeter). By combining the two, he invented aqua regia, one of the few substances that can dissolve gold. Besides its obvious applications to gold extraction and purification, this discovery would fuel the dreams and despair of alchemists for the next thousand years. He is also credited with the discovery of citric acid (the sour component of lemons and other unripe fruits), acetic acid (from vinegar), and tartaric acid (from wine-making residues). (reference)
Toxicity
Concentrated nitric acid and its vapors are highly corrosive to the eyes, skin, and mucous membranes. Dilute solutions cause mild skin irritation and hardening of the epidermis. Contact with concentrated nitric acid stains the skin yellow and produces deep painful burns. Eye contact can cause severe burns and permanent damage. Inhalation of high concentrations can lead to severe respiratory irritation and delayed effects, including pulmonary edema, which may be fatal. Ingestion of nitric acid may result in burning and corrosion of the mouth, throat, and stomach. An oral dose of 10 mL can be fatal in humans. (reference)
(back)

Connect with us
Contact us today