
 |
For many years, different individuals and organizations have been putting specimens out in the atmosphere in all kinds of tests and shapes. There has been an attempt to standardize some of these tests, as well as the method of reporting the results. When initiating corrosion tests in the atmosphere, these standardized procedures should be consulted and used whenever possible. There tend to be three general types of specimens:
Panels;
Tensile specimens; and
Stress corrosion cracking specimens.
The panels are usually in the form of sheets 10 cm wide, 15 cm long, and about 0.2 cm thick; the tensile specimens in a machined "dumb bell" shape to fit tensile testing machines; and the stress corrosion specimens in jigs to suit the type of stress being considered.
In addition to the atmospheric conditions at the test site the following factors are important aspects for the design and interpretation of atmospheric corrosion tests:
Shape of the specimen
Direction it faces
Amount of shelter, drip, or runoff from other specimens
Elevation
Shading
Unusual contamination.
Panel specimens are usually placed in racks at a 30-degree angle to the horizontal, facing the source of corrosive elements. They are electrically insulated from the racks on which they are mounted and are arranged so that drip from neighbouring panels does not contaminate them as illustrated in the following Figure.

Atmospheric test rack exposed to a marine West Coast environments. (Courtesy of Defence R&D Canada Atlantic)
Cylindrical specimens are mounted horizontally, facing the same direction as the panels. They may be exposed fully to the weather or be partially sheltered, depending upon the requirements of the test. There is a great variety of test fixtures and shape of specimens used for environmental cracking tests. The following Figures show respectively a full exposure and a semi-sheltered test stations equipped with TOW galvanic sensors, temperature, and SO2 level recorded with a data logger.

Outdoor exposure test station with specimen rack on top and data logger attached below at an Army Base in Northern Australia. (Courtesy of DSTO Australia)

Semi sheltered test station with test coupons, wet candle apparatus, and data logger attached at an Army Base in Northern Australia. (Courtesy of DSTO Australia)
The following Figure shows a custom built test rack designed to be mounted on a full exposure test station in order to reveal the atmospheric corrosion resistance of aluminum and magnesium alloys to crevice corrosion.
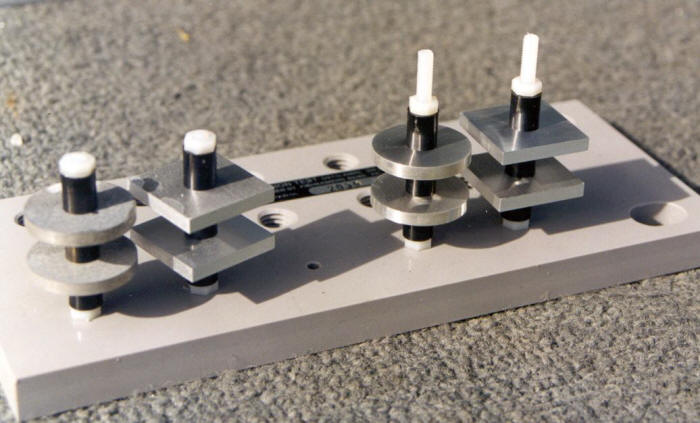
Aluminum and magnesium test specimens equipped with crevice spacers and mounted on a rack to be exposed at the previous test station. (Courtesy of DSTO Australia)
In most exposure tests, enough specimens are used so that removals may be made after periods of 1, 2, 7, and 20 years or 2, 5, 10, and 20 years. Such programs correct for materials that have changes in corrosion rate after the first one or two years. Very short-term tests usually can be misleading in that the condition of the metal surfaces during the first few days of exposure may affect the initial corrosion rate, or "average weather conditions" may not be encountered during the initial exposure period.
Some tests are continued until failure, as in SCC testing. However, in tests of protective coatings, as well as in others, periodic measurements of weight loss, pit depth, and change in tensile strength are made. All of these factors affect the duration of tests, but in most atmospheric exposures, a program covering a time span of ten years or more is often considered.
In many atmospheric corrosion tests, the specimens are observed every year for changes in appearance. At predetermined intervals, samples are removed, the weight loss is determined, tensile tests are made, and if pitting is significant, the deepest pit is measured, as well as a number of other pits, to determine average depth. In a few instances, composition of corrosion products is determined.
Results of corrosion tests are usually reported as:
Loss in weight or reduction in thickness per unit of time
Change in tensile strength
Time-to-fracture (SCC)
Time-to-perforation
Time-to-10%-increase-in-electrical-resistance
Time-to-initial-rust, 10% rust, or complete rust, or
In some cases, changes in ductility
The reduction in thickness or penetration in microns per year (Ám/y) or mils per year (mpy) is the most general and useful measurement of corrosion rate.
So far, reference has been made to natural exposures in the atmosphere. Many workers feel that evaluations are needed in times less than those required for on-site tests, and therefore have devised accelerated methods based on a previous determination of the dominant corrosion factors. The preferred practice is to design such tests to represent the most severe conditions for the corrosion mechanism involved.
However, it is useful to consider how realistically corrosion acceleration may be achieved. Raising the temperature can be useful but may cause changes in the form and nature of hydrous gels often important in the initial stages of corrosion. Increasing the concentration or corrosive agents in the salt spray, for example, may not necessarily be appropriate during cyclic testing since even an initially dilute spray will, after a sufficient number of cycles, result in the solubility of ionic species being exceeded.
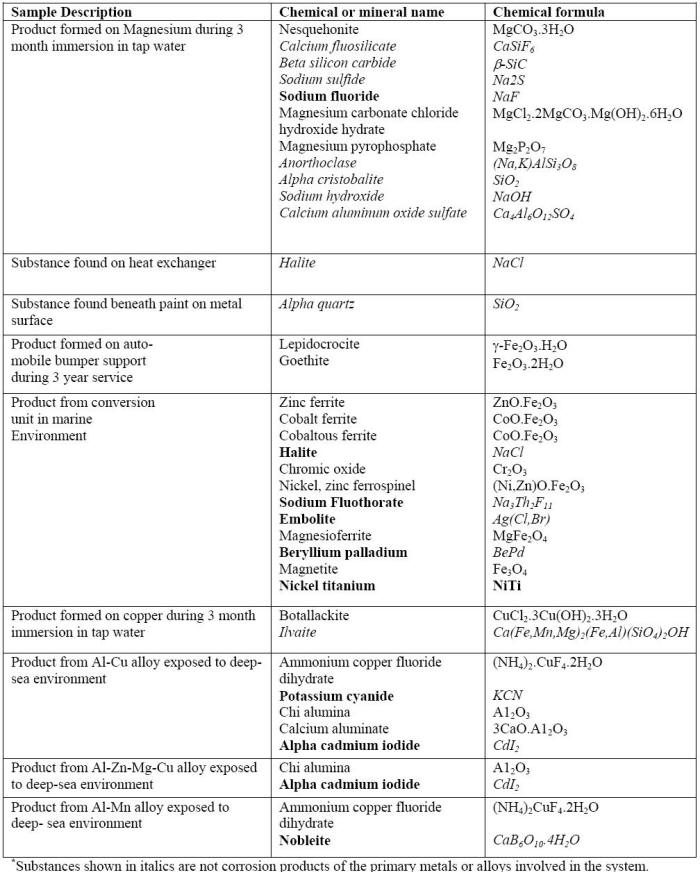
Generally, corrosion products developed by synthetic environments such as those produced in the ASTM B117 test are substantially different from those produced during natural weathering or even by wet-dry mixed salt spray tests. For example, corrosion of aluminum or zinc specimens in ASTM B117 primarily produces soluble species such as AlCl3 or ZnCl2 with little corrosion product remaining on their surfaces. Exposures in a wet-dry test, in contrast, cause the formation of corrosion products on those metals more representative of those formed during natural exposure. On aluminum, for example, hydrated alumina containing chloride and amorphous material are produced in both the high sulfate and high chloride cyclic salt spray tests. The reality can be even more complex as illustrated in the following Table in which it can be seen that the products found on specimens exposed to real environments often consist of corrosion products mixed with various foreign materials .
Results of X-Ray diffraction of products found specimens exposed to real environments.
| (previous) | Page 7 of 8 |
(next) |