
 |
The following sections contain some specific information on the atmospheric corrosion performance of the most popular systems used in normal atmospheric conditions.
Iron, in its various forms, is exposed to all kinds of environments. It tends to be highly reactive with most of them because of its natural tendency to form iron oxide. When it does resist corrosion it is due to the formation of a thin film of protective iron oxide on its surface by reaction with oxygen of the air. This film can prevent rusting in air at 99% RH, but a contaminant such as acid rain may destroy the effectiveness of the film and permit continued corrosion. Thicker films of iron oxide may act as protective coatings, and after the first year or so, could reduce the corrosion rate significantly as shown in the following Figure.
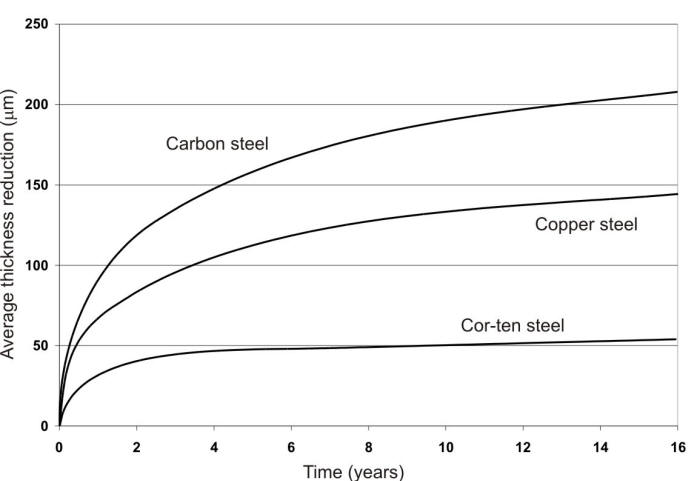
Time-corrosion curves of three steels in industrial atmosphere, Kearny, NJ: (1) ordinary steel; (2) Copper steel; and (3) Cor-ten.
While the corrosion rate of bare steel tends to decrease with time in most cases, the difference in corrosivity of different atmospheres for a particular product is tremendous. Similar ranges in corrosivity were determined by the ISO 9223 corrosion rates for steel. In a few cases, the corrosion rates of ferrous metals have been reported as increasing with time, and careful analysis of the exposure conditions generally reveals that an accumulation of contaminating corrosive agents has occurred, thus changing the severity of the exposure.
It is generally conceded that steels containing low amounts of copper are particularly susceptible to severe atmospheric corrosion. In one test over a 3 1/2-year period in both a marine and an industrial atmosphere, a steel containing 0.01 % copper corroded at a rate of 80 µm/y, whereas increasing the copper content by a factor of five reduced the corrosion rate to only 35 µm/y. Other tests comparing gray cast iron, malleable iron, and low-alloy steels indicated that their corrosion resistances were approximately the same.
Plain cast iron appears to have a corrosion rate about one half that of 0.2% copper steel in a marine atmosphere. One has to be careful in citing such differences to stipulate the composition of the carbon steel because corrosion behavior of carbon steels is influenced so markedly by small variations in copper and phosphorus content. In an industrial atmosphere, a structural carbon steel showed a penetration of about 20 µm, a copper structural steel about 10 µm, and a low-alloy steel about 4 µm after five years of exposure.
As indicated in ISO 9223 , it is impossible to give a corrosion rate for steel in the atmosphere without specifying the location, composition, and certain other factors. If one can relate exposure conditions to those described in the literature, a fairly good estimate can be made of the probable corrosion behavior of a selected material. However, all aspects of the exposure of the metal surface must be considered. A high-strength, low-alloy (HSLA) steel may show an advantage in corrosion resistance of 12:1 over carbon steel when freely exposed in a mild environment. As the severity or the physical conditions of exposure change, the HSLA steel will show less superiority, until in crevices or the backside of structural forms in a corrosive atmosphere, it will be no better than carbon steel.
Very little needs to be said about the behavior of stainless steels (Types 200 and 300), which contain high percentages of nickel and chromium, except that they can keep their shiny aspect without tarnishing for many decades as illustrated in “The Mudra”, shown in the following Figure , which was erected in the busy part of Toronto in 1974. The steels containing only chromium (Type 400) as the principal alloying constituent tend to rust superficially, but the others are relatively free from surface atmospheric corrosion. Many of them are susceptible to SCC in many common environments.

Toronto stainless steel sculpture the ‘Mudra’ by Ted Bieler completed in 1974. (Photo by Tom Skudra courtesy of the Nickel Institute)
Copper and its alloys are not exposed to the atmosphere in great quantities when compared with steel. However, this material brings aesthetic value to building construction, in addition to excellent corrosion resistance. The black and then green patina formed on the surface provides an attractive decorative finish, while sealing the metal from further corrosion. As a consequence, some copper has been used for roofs, gutters, and as flashings on wood or composition shingled roofs.
Find in your neighbourhood some structural elements made of copper and copper alloys and report on their state and condition.
Extensive tests have been made of the corrosion resistance of copper and its alloys to various atmospheres. Various alloys were exposed in rural, industrial, and marine atmospheres for periods of up to twenty years. From data accumulated in these tests and the calibrations of relative corrosivity of the test sites, a fairly clear picture can be obtained of the corrosion behavior of copper. In addition to the corrosion penetration rates, one must be mindful of dezincification of brasses and selective attack on some bronzes, as well as SCC illustrated by season cracking of brass. These types of corrosion contribute to the failure of the material in mechanical respects without significant weight changes or losses in thickness.
Where copper is used as flashing on roofs, corrosion has been encountered at the edge of the shingles as a continuous groove. This effect is more pronounced when the atmosphere contains both chlorides and sulfides, and with wood shingles as compared with roofs of other composition. Tests indicate that all-copper roofs 0.5 mm or more in thickness such as the roof in the following picture would last several centuries in an industrial atmosphere.

Green copper roof on Quebec Armory
If the green patina on copper alloys is desired for aesthetic reasons, pre-treatment of the surface with appropriate passivating solutions is recommended. If oxidation by sulfur compounds precedes the desired reaction, the surface will present only a dark brown color for many years.
Electrodeposited nickel and electroless nickel are widely used as a protective coating for atmospheric exposure, and some nickel alloys, while, selected for other reasons, are also exposed to atmospheric corrosion. The results shown in the following Table were obtained for several representative alloys. As can be concluded from the data, nickel tends to be passive in a marine atmosphere. The ratio between the corrosion rate for nickel exposed to the industrial atmosphere and that exposed to rural or marine atmospheres was 28:1.
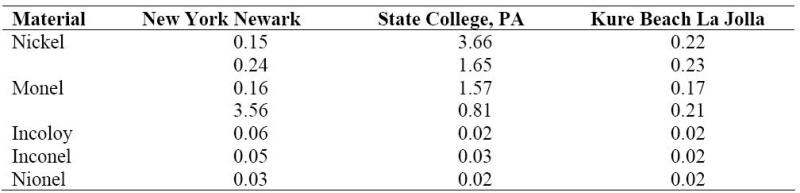
Corrosion in mm/year of nickel and its alloys in various atmospheres.
Aluminum, in its many forms is exceeded only by steel in tonnage directly exposed to the elements. It is produced in the form of wrought products, extrusions, and castings with a large variety of alloying elements to impart desired mechanical properties. The atmospheric corrosion behavior of aluminum products fits into some fairly well-defined patterns that are related to composition.
While pure aluminum has excellent atmospheric corrosion resistance and is used extensively as a cladding materials for this very reason, alloys containing copper and silicon as the principal alloying constituents are susceptible and should be used with care. In a rural atmosphere, the corrosion rate for most alloys is approximately 0.06 µm/y, with those containing large amounts of copper about double this low rate. Changes in tensile strength because of corrosion vary from 0 to less than 1% for sheet material.
In a marine environment, the differences between alloys appear as a tenfold increase, from about 0.6 µm/y for the less corrosion-resistant materials, to about 0.7 µm per year for the better materials. Pitting also is about 10 times greater in marine atmospheres. Corrosion can be much greater in a severe industrial atmosphere than in the marine atmosphere.
Some aluminum alloys develop severe pitting and a voluminous white corrosion product under some exposure conditions in a marine atmosphere. Aluminum roofs have been known to corrode severely at the overlaps. Some aluminum alloys also can be attacked in their intergranular regions when exposed after certain metallurgical treatments (cold working or precipitation hardening). General intergranular attack or exfoliation can then occur. The attack tends to start at sheared edges or punched holes, but is not restricted to these areas. Aircraft manufacturers, in particular, must guard against this type of corrosion.
In designing aluminum equipment, care must be exercised to avoid dissimilar metal couples and the attendant galvanic corrosion. Copper and rusty steel are particularly bad in contact with aluminum. Due to the passive film on stainless steel, it can be used in contact with aluminum in the atmosphere with little expectation of accelerated corrosion, despite the differences in potential. In addition, designers should be aware of the possibility that some aluminum alloys may be sensitized to intergranular corrosion by heat treatment.
As would be expected, constant exposure to moisture with a limited supply of oxygen to the aluminum surface leads to the rapid corrosion of any aluminum apparatus or equipment component. This is due to the highly reactive nature of aluminum that leads to formation of oxides or hydroxides. In the presence of oxygen, a protective aluminum oxide film develops on any aluminum surface. This oxide film is substantially unreactive with the normal constituents of the atmosphere. If the film is removed by mechanical or chemical means and the aluminum exposed to water, a rapid reaction sets in and large quantities of the aluminum are converted to the hydroxide and subsequently to the oxide.
Most of the 1000, 3000, and 6000 series of wrought alloys, the magnesium, and the silicon magnesium casting alloys are relatively immune to SCC in normal atmospheres. However, the other alloys may be susceptible under some conditions. This subject should be investigated carefully by the designer or user if stresses are high and the atmosphere is corrosive.
The usual corrosion behavior in the atmosphere involves pitting and roughening of the surface with a fairly large decrease in the corrosion rate after the first one to three years of exposure. In a marine atmosphere, the maximum pit depth might reach 150 mm within 2 years, yet not exceed 200 mm in 20 years. In an industrial atmosphere, initial pits might reach 4 mils in the first period and not exceed 125 mm at the end of 20 years. In terms of average corrosion rate, the initial rate would be about 0.08 mm/y, dropping to less than 0.3 mm/y by seven years.
Severe pitting has been encountered where aluminum surfaces were contaminated by either alkaline dust or coral dust containing chlorides, followed by condensation. On some of the South Pacific islands, dust collected from the surfaces of sheltered structures, such as those inside aircraft wings, contained 67% chloride by weight.
In the design of aluminum structures, the usual precautions of avoiding crevices or pockets and coupling with dissimilar metals must be observed. An example of this is in the overlapping encountered between aluminum roofs and siding. In some marine or industrial atmospheres, the aluminum perforated at the laps within a few months. In addition, stress concentrations should be avoided, such as those found in some riveted structures, in the vicinity of welds, and at notches or inside corners. Where it is impractical to avoid dissimilar metals, the aluminum should be electrically insulated from the more noble metal by means of washers, sleeves, etc. In some instances, covering the noble metal with an organic finish is sufficient to greatly reduce galvanic couple corrosion.
To illustrate the problems encountered in designing aluminum structures, an amusing and fairly well-known, yet illustrative story comes to mind. A new processing plant was built having an un-insulated aluminum roof attached directly to steel support frames. This was known to have worked well in several instances. However, the high RH in the plant caused condensation on the cool roof during rainstorms, evenings, and cold spells in the winter.
Zinc is exposed to the atmosphere in the form of galvanized sheet, as in flashings on roofs; as die castings, and as coatings on steel, either hot dipped or electroplated. Studies of the corrosion of zinc in the New York atmosphere indicated that there was less than a 10% difference between the corrosion rate of galvanized iron, zinc die castings, and three grades of rolled zinc. It was also found that the corrosion rate tended to be a linear function with time. In some instances, where the rate changed after a period of time, it was concluded that the amount of contamination in the atmosphere had changed.
The general behavior of zinc metal and zinc coatings is described in the ISO tables presented earlier. Note the particularly low rates of attack on zinc as compared with steel in marine exposures where chloride deposition is important. Such excellent resistance is acquired by the hard, dense, protective products of corrosion in a chloride atmosphere. Similar results cannot be obtained in a sulfurous atmosphere where the products are soft, voluminous, and non-protective.
Find in your neighbourhood some structural elements made of galvanized steel and report on their state and condition.
Zinc-base die castings usually are not exposed boldly to the outside atmosphere without a protective coating. When breaks or pits occur in coatings such as nickel and chromium, the corrosion of the die casting may be accelerated due to the dissimilar metal contact and thus give a false impression of the corrodibility of zinc. Many small parts of machinery, household appliances, and hardware are made of zinc-base die castings or "white metal” and are exposed to an indoor atmosphere where their corrosion behavior is very good (White metal is typically 93 to 96% Zn, 4% Al, 0.05% Mg, and sometimes 1 to 3% Cu). In these cases where severe corrosion is encountered in this relatively mild atmosphere, the cause may be improper alloy selection or the use of material containing too high a percentage of impurities.
Galvanized steel is the most important application of zinc. Worldwide, the use of zinc for galvanizing results in an annual consumption of more than three million tons of zinc, constituting nearly one-half of the world zinc production. Most of these are hot-dipped galvanized coatings containing a small amount of aluminum. Thickness of electroplated coatings is considerably lower than those applied by the hot dip process. Galvanizing produces a zinc coating on the steel surface and is one of the most effective methods for corrosion protection of steel. This is attributed to the excellent corrosion resistance of zinc coatings, particularly in atmospheric environments.
Corrosion rates for zinc, like other metals can vary by as much as two orders of magnitude in atmospheric environments depending on the specific environmental conditions. Therefore, it is important to know the specific corrosion rate in a given application environment in order to effectively use zinc coated steels in outdoor structures. The most commonly used method for corrosion life estimation of galvanized steels has been the use of generalized values for the different types of atmospheres as shown in the following Figure.
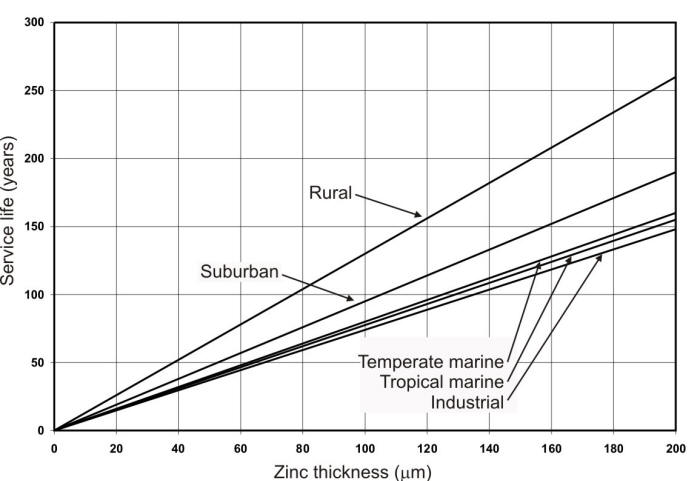
Service life of hot-dip galvanized coatings as a function of zinc thickness and specific environments.
This method uses a generalized value to represent the corrosion rates for five predetermined atmospheric environments as a function of zinc coating thickness. Service life in Figure 9.43 is defined as the time to 5% rusting of the steel surface. It can be used to estimate the service life of a given coating thickness or to specify a coating for a given environment.
Use one of the corrosivity maps to specify the thickness required of a galvanized coating to achieve a useful life of fifty years in the various environments described on that map.
The method is applicable to zinc-coated steel produced by batch or continuous galvanizing. It is applicable to hot-dip, electrogalvanized, and thermal sprayed coatings. However, it does not apply to coatings containing alloying elements larger than 1%. The method assumes that the galvanized product is free of significant defects that could accelerate corrosion. Additionally the service life prediction does not consider issues of water entrapment that can create severe crevice chemistry.
Many other alloys are exposed to the atmosphere under a variety of circumstances. The resulting corrosion problems tend to be specific. Lead and tin alloys tend to be inert in the atmosphere unless some specific type of contamination exists. As far as exposure to the atmosphere is concerned, tin appears mostly as a coating on steel containers where it can accelerate the corrosion of the more anodic steel by pitting. Lead is sometimes used on roofs, but more often is found indoors in chemical plants.
Due to their inertness, silver and gold are used as alloys to confer corrosion resistance to more reactive metals. Silver reacts with sulfur compounds in the atmosphere to produce heavy coatings of black silver sulfide. This corrosion product is soft and rubs off of electrical contacts.
Titanium alloys have very good corrosion resistance in the atmosphere, as do the iron-nickel chromium alloys. There are many alloys that hold a specific place in the metal family, such as pewter, speculum, constantan, and the like, which possess certain atmospheric corrosion resistance properties, but are used so infrequently that they do not warrant general discussion.
Any discussion of atmospheric corrosion should include consideration of the corrosion behavior of various metallic protective and decorative coatings. These are usually divided into two general categories:
Coatings that confer protection to the basic metal, and
Coatings that are used for decorative purposes.
As protective coatings, a distinction must be made between those such as zinc, cadmium, and aluminum on steel that protect by sacrificial behavior, and those that must provide a substantially continuous protective envelope around the protected metal. In the latter category are metals such as nickel, tin, silver, brass, and chromium.
Sacrificial metals corrode as coatings in much the same manner as they do as solid metals until the base or protected metal is exposed at pores or bared areas. The galvanic couple effect then begins to accelerate the corrosion of the protective coating. This galvanic couple effect tends to protect the base metal at the pores or bared spots. General corrosion of the sacrificial type of coating tends to follow a linear function that is peculiar to the particular site.
This reaction is in contrast to what happens in the case of the more noble protective coatings that act as an envelope and resist the atmosphere due to their inertness, passivity, or protective films. As soon as a pore or bare spot appears, the corrosion of the base metal is accelerated. Die castings of a metal such as zinc covered with a copper-nickel-chromium coating will suffer severe corrosion at large pores or discontinuities in the coating.
While most metal coatings are applied by electroplating, some are produced by flame or plasma spraying, hot dipping, electrostatic sputtering, or vapor deposition. The corrosion rate of a metal coating is largely independent of the method of application, except where impurities play a role. Powders of various metals have been attached to the surface of a base metal by either organic or inorganic binders. Where these metal particles are in mutual contact, and in contact with the base metal, they can perform much like a metallic coating. In noble types of coating, porosity is very important and tends to decrease rapidly as the coating thickness increases beyond 13 mm. No clear cut information is available on the factors that influence porosity in electrodeposited coatings.
Essentially all polymers freely exposed to the elements will change in some manner. The active rays of the sun become potent agents of change in the organic materials. Further polymerization of the resin can occur to produce embrittlement. Other types of new bonding can be triggered to make polymers more crystalline. Any volatile component of the material, such as a plasticizer, can be evaporated. The polymer chains may be simply oxidized and broken up to destroy the product. Oxygen, ozone, and moisture act with the sunlight to degrade the plastics as illustrated in the following Figure.

Plastic material degraded over a few years of exposure to sun rays on a window sill.
The external evidence of attack may be blushing (loss of gloss), chalking, or change in color of the product. This is often observed on the epoxy and polyester polymers when they have been boldly exposed to the environment. However, only mechanical tests will reveal the extent of degradation of either thermoplastic or thermosetting resins. The effect of high atmospheric temperatures or heating from direct exposure to the sun can be particularly severe on thermoplastic polymers. Creep or distension of the polyvinyl chloride and polyethylene plastics will occur readily unless provision is made to prevent overheating or stressing of the materials. Certain other thermoplastics can be dimensionally stable under normal atmospheric temperatures. The strength of these thermosetting resins is not noticeably changed.
Polymeric materials should be thoroughly tested if they are to be exposed freely in the atmosphere. ASTM Recommended Practice D1435 describes the appropriate conditions for such a test exposure and suggests tests that might be used to evaluate changes in the materials. Changes in mechanical and physical properties of the polymers are determined for definitive results. Weight gains or losses can be of interest, but do not provide substantive results. Accelerated cabinet testing of the materials can be performed with essentially the same validity as when testing metallic materials. Poor materials can be eliminated, but extrapolation of the data to forecast the life of plastic parts in the atmosphere should not be attempted. ASTM D1499, D 2565, and G26 may be used to ensure the controlled cabinet testing of the plastics. ASTM D750 should be used for the evaluation of the elastomers.
The preventive measures used to ensure the integrity of polymeric materials are similar to those for metals, i.e., the exterior should be covered. The exterior surfaces are painted or metal plated to provide a barrier between the material and the atmosphere. Alloying of the basic material with small amounts of other resins can often upgrade the stability of the material.
| (previous) | Page 8 of 8 | (end) |