Module Four of CCE 281 Corrosion: Impact, Principles, and Practical Solutions
Iron E-pH (Pourbaix) Diagram
The following Figure illustrates the E-pH diagram for iron in the presence of water or humid environments at 25oC, which was calculated by considering all possible reactions associated with iron in wet or aqueous conditions listed in the Table below, excluding therefore drier forms of corrosion products such as magnetite (Fe3O4) or iron (ferric) oxide (Fe2O3).
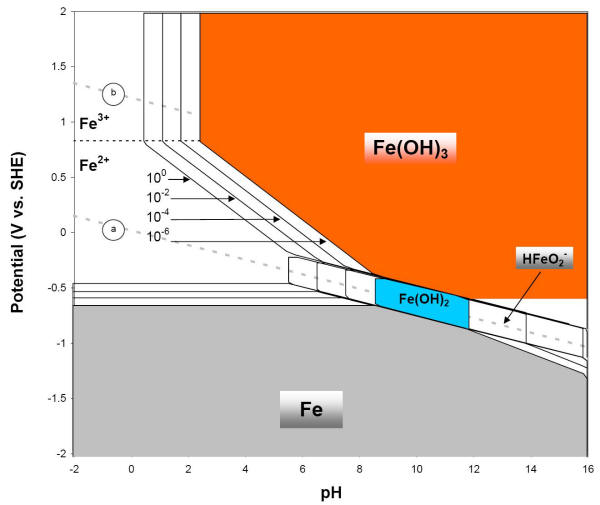
E-pH diagram of iron or steel with four concentrations of soluble species, three soluble species and two wet corrosion products (25oC)
Possible reactions in the Fe-H2O system between the species most stable in wet conditions
The various stability regions for these drier corrosion products are shown in the following Figure where the predominant compounds and ions are also indicated.
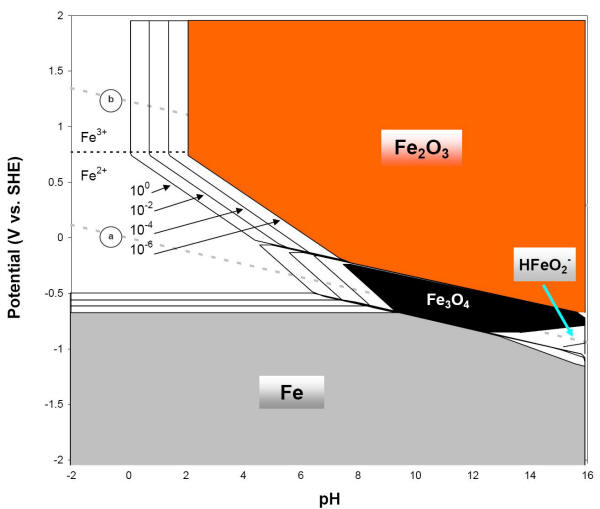
E-pH diagram of iron or steel with four concentrations of soluble species, three soluble species and two dry corrosion products (25oC)
At potentials more positive than -0.6 and at pH values below about 9, ferrous ion (Fe2+ or Fe II) is the stable substance. This indicates that iron will corrode under these conditions. In other regions of the iron E-pH diagram, it can be seen that the corrosion of iron produces ferric ions (Fe3+ or Fe III), ferric hydroxide [Fe(OH)3], ferrous hydroxide [Fe(OH)2], and at very alkaline conditions, complex HFeO2- ions. The solid corrosion products considered are different than earlier, ferric oxide (Fe2O3) and magnetite (Fe3O4), both important iron ore constituents.
The presence of a relatively large immunity region in the previous Figures, where corrosion products are solid and possibly protective, indicates that iron may corrode much less under these potential/ pH conditions.
These diagrams also indicate that if the potential of iron is made sufficiently negative or shifted cathodically below approximately -0.5 V vs. SHE in neutral or acidic environments, as indicated in the following Figure, iron will corrode much less. This explains the generally accepted cathodic protection criterion of -0.85 V vs. CCSRE used across industries to protect steel assets buried in soils. The difference between this cathodic potential and line is indicative that such potential will also tend to electrolyze water into hydrogen as indicated in equations.
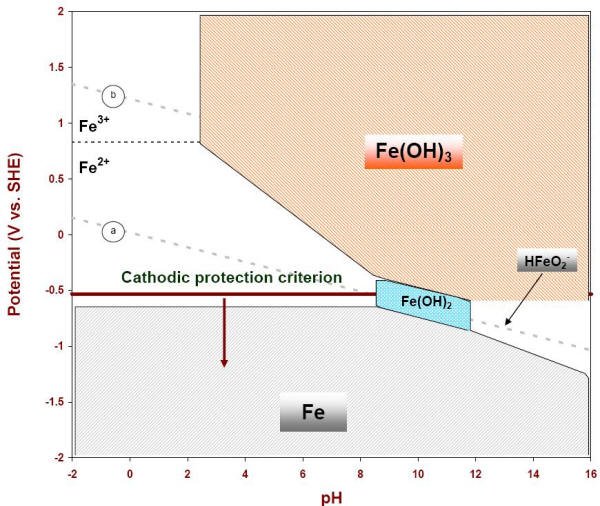
E-pH diagram of iron with the cathodic protection criterion at
-053 V vs. SHE (-0.85 V vs. CCSRE)
Fortunately a few software systems are available to compute E-pH diagrams.
| (previous) | Page 17 of 17 | (next Module) |
See also: Equilibrium reactions of iron in water, Iron corrosion products, Iron species and their thermodynamic data, Rust chemistry, Rust converters, Steel corrosion

Connect with us
Contact us today