Dobereiner's Periodic Table

Döbereiner, Johann Wolfgang (Germany, 1780-1849)
During the 1820s Döbereiner’s experiments with the ignition of hydrogen on contact with powdered platinum led the Swedish chemist J.J. Berzelius to develop the concept of catalysis. Toward the end of the decade Döbereiner found that the properties of bromine, a liquid, seem halfway between those of chlorine gas and the solid iodine. He recalled a comparable graduation of properties in two other sequences, i.e. calcium, strontium, barium; and sulfur, selenium, tellurium. Each of Dobereiner's triads was a group of three elements. The appearance and reactions of the elements in a triad were similar to each other. He showed that in each triad the mean of the lightest and heaviest atomic weights approximated the atomic weight of the middle element. But he could not substantiate his hypothesis with a sufficient number of triads, and his findings were regarded in his time as merely interesting curiosities.
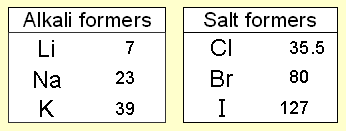
Atomic masses At this time, scientists had begun to find out the relative atomic masses of the elements. Dobereiner discovered that the relative atomic mass of the middle element in each triad was close to the average of the relative atomic masses of the other two elements. This gave other scientists a clue that relative atomic masses were important when arranging the elements.
Between 1829 and 1858 a number of scientists (Jean Baptiste Dumas, Leopold Gmelin, Ernst Lenssen, Max von Pettenkofer, and J.P. Cooke) found that these types of chemical relationships extended beyond the triad.
See also: Development of the Periodic Table, de Chancourtois, Dobereiner, Mendeleev, Moseley, Newlands, Seaborg

Connect with us
Contact us today