Moseley's Periodic Table

Henry Gwyn Jeffreys Moseley (23 November 1887 – 10 August 1915)
Mendeleev's table was nine tenths of the way there, but needed one important modification before it became the modern periodic table - the use of atomic number as the organizing principle for the periods. According to Moseley, similar properties recur periodically when elements are arranged according to increasing atomic number. Atomic numbers, not weights, determine the factor of chemical properties.
Mendeleev ordered his elements in order of their relative atomic mass, and this gave him some problems. For example, iodine has a lower relative atomic mass than tellurium, so it should come before tellurium in Mendeleev's table - but in order to get iodine in the same group as other elements with similar properties such as fluorine, chlorine and bromine, he had to put it after tellurium, so breaking his own rules. In his invention of the Periodic Table of the Elements, Mendeleev had interchanged the orders of a few pairs of elements in order to put them in more appropriate places in this table of the elements. For example, the metals cobalt and nickel had been assigned the atomic numbers 27 and 28, respectively, based on their known chemical and physical properties, even though they have nearly the same atomic masses. In fact, the atomic mass of cobalt is slightly larger than that of nickel, which would have placed them in backwards order if they had been placed in the Periodic Table blindly according to atomic mass.
Moseley's experiments in X-ray spectroscopy showed directly from their physics that cobalt and nickel have the different atomic numbers, 27 and 28, and that they are placed in the Periodic Table correctly by Moseley's objective measurements of their atomic numbers. Using atomic number instead of atomic mass as the organizing principle was first proposed by the British chemist Henry Moseley in 1913, and it solved anomalies like this one. Iodine has a higher atomic number than tellurium - so, even though he didn't know why, Mendeleev was right to place it after tellurium after all!
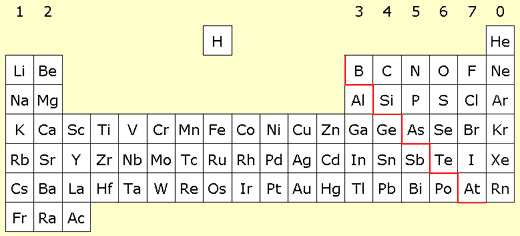
Metals and non-metals
More than three quarters of the elements in the modern table are metals. They are mainly found in the two left-hand columns (Groups 1 and 2) and the central block (the transition metals). Less than a quarter of the elements are non-metals, and are found on the right-hand side of the periodic table.
Early Death
When World War I broke out in Western Europe, Moseley left his research work at the University of Oxford behind to volunteer for the Royal Engineers of the British Army. Moseley was assigned to the force of British Empire soldiers that invaded the region of Gallipoli, Turkey, in April 1915, as a telecommunications officer. Moseley was shot and killed during the Battle of Gallipoli on 10 August 1915, at the age of 27. Experts have speculated that Moseley could have been awarded the Nobel Prize in Physics in 1916, if he had not been killed. As a consequence, the British government instituted new policies for eligibility for combat duty.
See also: Development of the Periodic Table, de Chancourtois, Dobereiner, Mendeleev, Moseley, Newlands, Seaborg

Connect with us
Contact us today