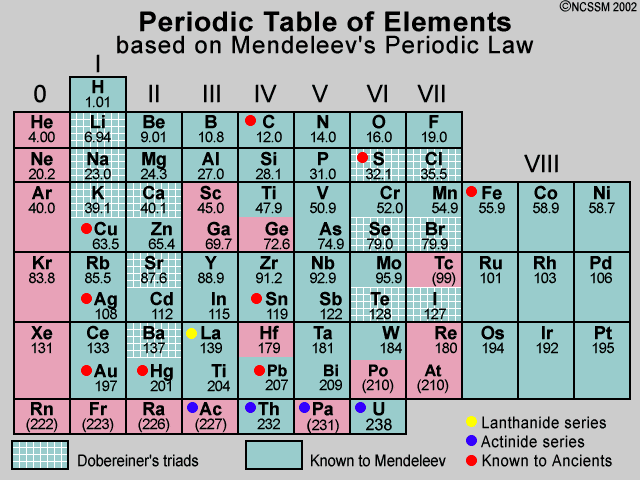
Mendeleev's Periodic Table

In 1869, just five years after John Newlands put forward his Law of Octaves, a Russian chemist called Dmitri Mendeleev published a periodic table. Mendeleev also arranged the elements known at the time in order of relative atomic mass, but he did some other things that made his table much more successful.
- Indestructibility of Matter
- Mendeleev's Opinion on Global warming
- Mendeleev eight periodic principles
- Mendeleev on the law of periodicity
- Primary Object of Chemistry
- Principles of Chemistry by Dmitrii Mendeleev
- Rusting of Metals
Mendeleev realized that the physical and chemical properties of elements were related to their atomic mass in a 'periodic' way, and arranged them so that groups of elements with similar properties fell into vertical columns in his table.
Gaps and predictions Sometimes this method of arranging elements meant there were gaps in his horizontal rows or 'periods'. But instead of seeing this as a problem, Mendeleev thought it simply meant that the elements which belonged in the gaps had not yet been discovered. He was also able to work out the atomic mass of the missing elements, and so predict their properties. And when they were discovered, Mendeleev turned out to be right. For example, he predicted the properties of an undiscovered element that should fit below aluminum in his table. When this element, called gallium, was discovered in 1875 its properties were found to be close to Mendeleev's predictions. Two other predicted elements were later discovered, lending further credit to Mendeleev's table.
Modern day periodic tables are expanded beyond Mendeleev's initial 63 elements. Most of the current periodic tables include 108 or 109 elements. It is also important to notice how the modern periodic table is arranged. Although we have retained the format of rows and columns, which reflects a natural order, the rows of today's tables show elements in the order of Mendeleev's columns. In other words the elements of what we now call a 'period' were listed vertically by Mendeleev. Chemical 'groups' are now shown vertically in contrast to their horizontal format in Mendeleev's table. (reference)
It is also worthy to note that Mendeleev's 1871 arrangement was related to the atomic ratios in which elements formed oxides, binary compounds with oxygen whereas today's periodic tables are arranged by increasing atomic numbers, that is, the number of protons a particular element contains. Although we can imply the formulas for oxides from today's periodic table, it is not explicitly stated as it was in Mendeleev's 1871 table. The oxides ratio column was not shown in earlier Mendeleev versions.
Mendeleev rewrote each edition of Principles of Chemistry, including all new scientific data-particularly confirmations of the periodic law-and reanalyzing difficulties that had arisen to hinder its confirmation (inert gases, radioactivity, radioactive and rare-earth elements)"
See also: Development of the Periodic Table, de Chancourtois, Dobereiner, Mendeleev, Moseley, Newlands, Seaborg

Connect with us
Contact us today